An Alternative Option in EtO Testing
New regulations and events in recent weeks have put a spotlight back on the issue of ethylene oxide (EtO) and the risks and concerns associated with public welfare.
Heightened awareness of EtO issues and strict regulation have subsequently led stack testers and industry personnel to the question of how to more effectively and precisely quantify EtO. More stringent limits demand lower detection limits. The industry has expanded since original regulations were enacted, creating increased EtO loading and making original test requirements and scopes more of a ‘square peg, round hole’ situation.
“What are my options?” “Are there more options than just those listed in the regs?” “What technology works best for my application?” These are just some of many questions stack testers and industry personnel are asking themselves right now.
Traditionally, the industry has turned to EPA Reference Method 18 (40 CFR 60) as its primary measurement option for EtO, but there might be a more viable alternative primed to seize a slice of the ol’ EtO testing pie. Below I discuss the more traditionally used Method 18 but also introduce an up-and-coming alternative– EPA Method 320, or more specifically, an optimized EPA Method 320.
Recent Updates…
On May 29, 2020, the EPA finalized a revision to the National Emission Standards for Hazardous Air Pollutants (NESHAP) for the Miscellaneous Organic Chemical (MON) manufacturing industry. This broad air toxics rule includes new provisions that specifically aim to reduce EtO emissions from storage tanks, process vents, and equipment leaks by 0.76 tons per year (tpy) by:
• requiring 99.9% removal efficiency from process control devices,
• reducing concentrations in air emissions to less than 1 ppm, or
• reducing air emissions to less than 5 pounds per year (lb/yr).
These requirements directly impact commercial sterilization facilities. On June 5th, the EPA proposed an Information Collection Request (ICR) for select EtO commercial sterilization facilities with the goal of better grasping the EtO issue at hand.
These actions taken by the EPA come on the heels of increasing public scrutiny and pressure on EtO associated industries. Regulators have come down hard on companies to increase EtO mitigation. In some cases they have gone as far as to cease operations entirely. As knowledge about elevated cancer risk traced back to EtO exposure becomes more prevalent, community activist organizations like Stop EtO in Illinois have sprung up, carrying with them a louder voice.
Traditional Measurement Option – EPA Method 18
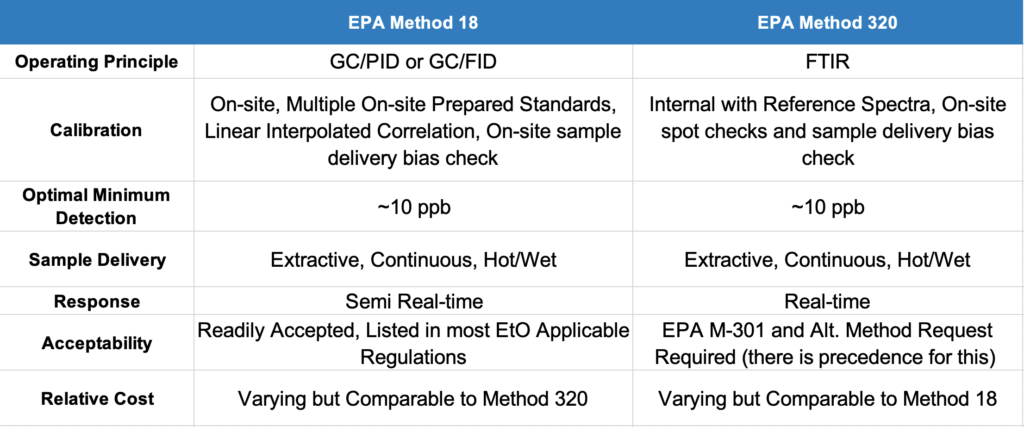
USEPA Method 18, “Measurement of Gaseous Organic Compound Emissions by Gas Chromatography,” is the most widely used method in determining EtO concentrations. Method 18 is a tried-and-true method that, given the right circumstances, can reach detection levels on the order of 10 ppb. Method 18, amongst other regulations, is specifically listed as an option for testing in USEPA 40 CFR 63 Subpart O “Ethylene Oxide Emissions Standards for Sterilization Facilities,” and USEPA 40 CFR 63 Subpart FFFF “Miscellaneous Organic Chemical Manufacturing” (a.k.a. MON), for example. For reference, in case you are more familiar with CARB Method 431, Method 18 is very similar except it includes more compounds than just EtO.
Method 18 utilizes gas chromatography (GC) to separate different constituents in the sample gas stream. GC is usually operated in tandem with various detectors; typically, a flame ionization detector (FID) or a photoionization detector (PID) for EtO.
In EtO applications, Method 18 is most commonly conducted in an ‘online’ manner. A sample is extracted from the source at a constant rate through a heated delivery system and delivered to the heated GC sample manifold where it is ‘injected’ in varying time segments. The timing depends on how long it takes an injection to make it through the GC column. Because it can take upwards of 10-15 minutes for this to occur, the results are considered to be collected in ‘semi real-time’.
The ‘online’ method is recommended for EtO applications due to the compound’s relatively high reactivity. EtO has the potential to completely disappear in a Tedlar bag within 36 hours. It is not uncommon for line-losses in the sample line used to transport the gas samples to the instrumentation to routinely lose 25% of the original EtO before making it to the GC.
Gas chromatography is practical to measure only a couple of constituents at a time before data quality is affected with overlapping peaks and varying column release time segments. With the GC, you have to know in advance exactly what you are looking for. GC also must be specifically calibrated for each target constituent on-site using prepared standards. This can be time-consuming and leads to an inherent risk of standard degradation, which would affect calibration and, ultimately, data quality.
GC cannot measure O2, CO2, and H2O, which means other methods must be utilized in conjunction with Method 18 in order to obtain a gas molecular weight. This is important because the molecular weight of the gas is required to calculate mass emission-based removal efficiencies (required in most applications).
Alternative Measurement Option – EPA Method 320
EPA Method 320, “Measurement of Vapor Phase Organic and Inorganic Emissions by Extractive Fourier transform infrared (FTIR) Spectroscopy” is increasingly being used by stack testers. It is also gaining preference by regulators as an alternative to Method 18 for EtO testing.
Method 320 utilizes an FTIR Spectrometer to obtain a spectrum of the sample gas by passing an infrared beam through the sample as it flows through a gas cell. After identification of the compounds from the infrared spectrum of a sample mixture, the concentrations are measured by comparing band intensities in the sample spectrum to those in reference spectra of the compound.
Sampling for Method 320 is conducted in a similar manner as the on-line version of Method 18. A sample is extracted from the source at a constant rate through a heated transport system and delivered to a heated FTIR. The major difference between the two methods is in the analytical instrumentation. Once the FTIR instrument is set up on-site, the user does a spot check with a calibration transfer standard (CTS) and follows this with a dynamic spike (typically with a target analyte). At this point, the FTIR is essentially ready to receive and analyze a sample.
The FTIR approach has several positive attributes:
• Reference spectra are readily available for hundreds of compounds. For example, MKS Instruments, the manufacturer of the widely used MKS MultiGas 2030FTIR, provides an extensive library of reference spectra that can be input into the FTIR analysis method (or “recipe”) and used to quantify those compounds in the gas sample. These reference spectra, which include CO2 and H2O, essentially act as an internal calibration. This avoids the reliance upon on-site prepared standards and the associated risk of degradation.
• FTIR analysis can achieve very low detection levels. By itself, it can reach minimum detection limits (MDLs) of less than 100 ppb. With the addition of add-on technologies such as MAX Analytical’s Starboost optical filter, the method allows the operator to reach levels of EtO detection on the order of 10 ppb. This optimization of the standard method is a key to the successful utilization of Method 320 for many EtO sources subject to the MON NESHAP, such as sterilization facilities.
• The FTIR algorithm allows for sample data within 30 seconds, making it much more of a real-time sampling method than traditional GC analysis.
• The FTIR measures CO2 and H2O along with the target analytes making it easier to obtain a sample gas molecular weight. Due to their chemical structure, the FTIR cannot measure diatomic compounds (H2, O2, N2); however, it can be operated in series or parallel with other instrumentation like an O2 analyzer, MicroGC, etc.
• A unique attribute of the FTIR method is the ability to quantify compounds retroactively by re-analyzing the data. This means that, once you obtain a set of valid and representative sample spectra on-site, you can optimize and fine-tune the data at any time afterwards. This can include quantification of compounds that were not included on the original target compound list.
At present, there is one major caveat with use of Method 320.The method is currently not listed as an option in the regulations. Therefore, before you can use Method 320 for regulatory testing, an EPA Method 301 validation has to be performed on the given source type and an alternative method request must be approved by the applicable administrator before testing.
These are not insignificant undertakings. However, the upside of this additional work is that the end-user will benefit from a method and operating principle that best suits their needs.
Wrapping up…
By now you can tell what way I would ever so gently lean if I were planning an EtO test program – but hey, I like what I know. Despite my preference for Method 320, it’s important to know there are alternative options for measuring EtO. Recent finalization of revisions to the MON NESHAP, EPA’s ICR for commercial sterilization facilities, and heightened public scrutiny have raised the stakes in EtO regulation, mitigation, and transparency. Since there are several factors that go into an optimal sampling approach for a given facility, front end consulting and project design with an experienced resource is as crucial now more than ever.
The author of this blog post is an emissions test project manager that has been involved in EtO state of affairs over the past 18 months and has gained insight into the issues and associated sampling solutions. Accumulating ample experience in FTIR over the span of nearly a decade, he has planned and led multiple Method 320-based EtO test programs, validated (via Method 301) the optimized method at a commercial sterilization facility, and has drafted and received approval from the EPA for an alternative method request. For more EtO insight, contact Kenny Sullivan at ksullivan@cleanair.com or give him a call at 847-654-4527.